Covid 19 Vaccine Pregnant Fda
Before the US. The FDA is not involved in COVID-19 vaccine distribution or appointments.
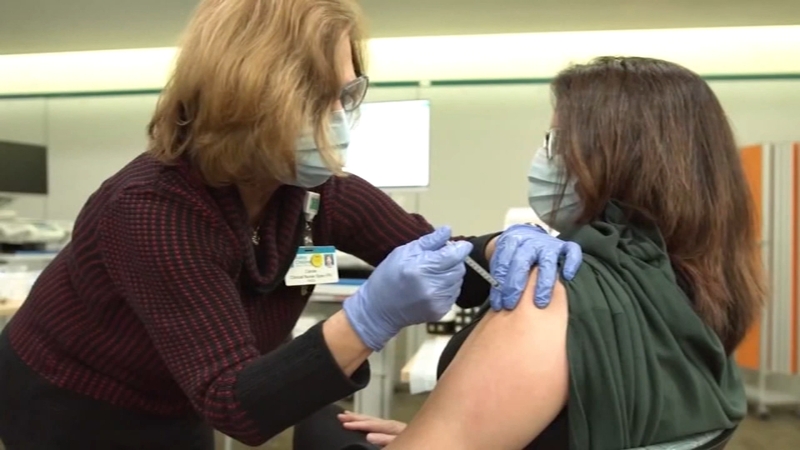 Valley Fertility Experts Weigh In On Covid 19 Vaccine Abc30 Fresno
Valley Fertility Experts Weigh In On Covid 19 Vaccine Abc30 Fresno
Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of coronavirus disease 2019 COVID-19 caused by.

Covid 19 vaccine pregnant fda. On December 11 2020 the US. State-Level Data Report December 2020. Food and Drug Administration should be made available to pregnant women who choose to.
The vaccine has not yet been studied in pregnant women. An FDA committee meets Friday to review the one-dose COVID-19 vaccine from JJ. The Food and Drug Administration will likely recommend against giving the Pfizer vaccine to pregnant women according to a report.
When making a decision pregnant people and their healthcare providers should consider the level of COVID-19 community transmission the patients personal risk of contracting COVID-19 the risks of COVID-19 to the patient and potential risks to the fetus the efficacy of the vaccine the side effects of the vaccine and the lack of data. CDC and the Food and Drug Administration FDA have safety monitoring systems in place to capture information about vaccination during pregnancy and will closely monitor reports. Food and Drug Administration issued the first emergency use authorization for a vaccine for the prevention of coronavirus disease 2019 caused by severe acute.
American Academy of Pediatrics Letter to HHS and FDA Children in COVID-19 Vaccine Trials September 2020. Food and Drug Administrations Vaccines and Related Biological Products Advisory Committee unanimously voted Friday to recommend Johnson Johnsons. On December 11 2020 the US.
Read guidelines here. Go to the CDC website to see how and when you can get vaccinated in your state. The FDA issued an emergency use authorization EUA for the vaccine on Dec.
The World Health Organization WHO recently updated its guidance to say pregnant people at high risk of exposure to COVID-19 and those at risk of severe disease should be vaccinated. On December 18 2020 the US. Centers for Disease Control and Prevention Coronavirus COVID-19 2020.
Authorization for its use in the USA is likely to swiftly follow. Yes COVID-19 vaccines currently authorized by the Food and Drug Administration FDA should not be withheld from pregnant individuals who choose to receive the vaccine. In addition women do not need to avoid getting pregnant after receiving Pfizers COVID-19 vaccine according to the CDC.
We strongly recommend that women talk with their doctor to discuss all factors about the vaccine and their pregnancy. For each COVID-19 vaccine authorized under an Emergency Use Authorization EUA the Food and Drug Administration FDA requires that vaccine recipients or their caregivers are provided with certain vaccine-specific EUA information to help make an informed decision about vaccination. MRNA vaccines do not contain the live virus that causes COVID-19 and therefore cannot give someone COVID-19.
At the time of this publication two vaccines developed for the prevention of COVID-19 have received EUA from the FDA. The American College of Obstetricians and Gynecologists ACOG said COVID-19 vaccines authorized by the US. Over the weekend after initial fears that pregnant and lactating health-care workers would be barred from receiving the COVID-19 vaccine because of a lack of safety data from clinical trials the.
The United States FDA has made the Moderna COVID-19 Vaccine available under an emergency access mechanism called an EUA. However COVID-19 vaccines are rapidly emerging and additional EUAs are likely to materialize. The EUA is supported by a Secretary of Health and Human Services HHS.
While pregnancy puts women at higher risk of severe COVID-19 very little data are available to assess vaccine safety in pregnancy WHO said in a statement. Food and Drug Administration issued the first emergency use authorization EUA for a vaccine for the prevention of coronavirus disease 2019 COVID-19 caused by. Pregnant women or nursing moms who want the COVID-19 vaccine should get one experts say.
Women who are pregnant breastfeeding or planning a pregnancy are. ACOG will strive to update this guidance as quickly as possible while maintaining accurate evidence-based information. American Academy of Pediatrics Children and COVID-19.
 Texas Covid 19 Vaccine Communication Tools
Texas Covid 19 Vaccine Communication Tools
 The Moderna Covid 19 Vaccine Coronavirus Immunization
The Moderna Covid 19 Vaccine Coronavirus Immunization
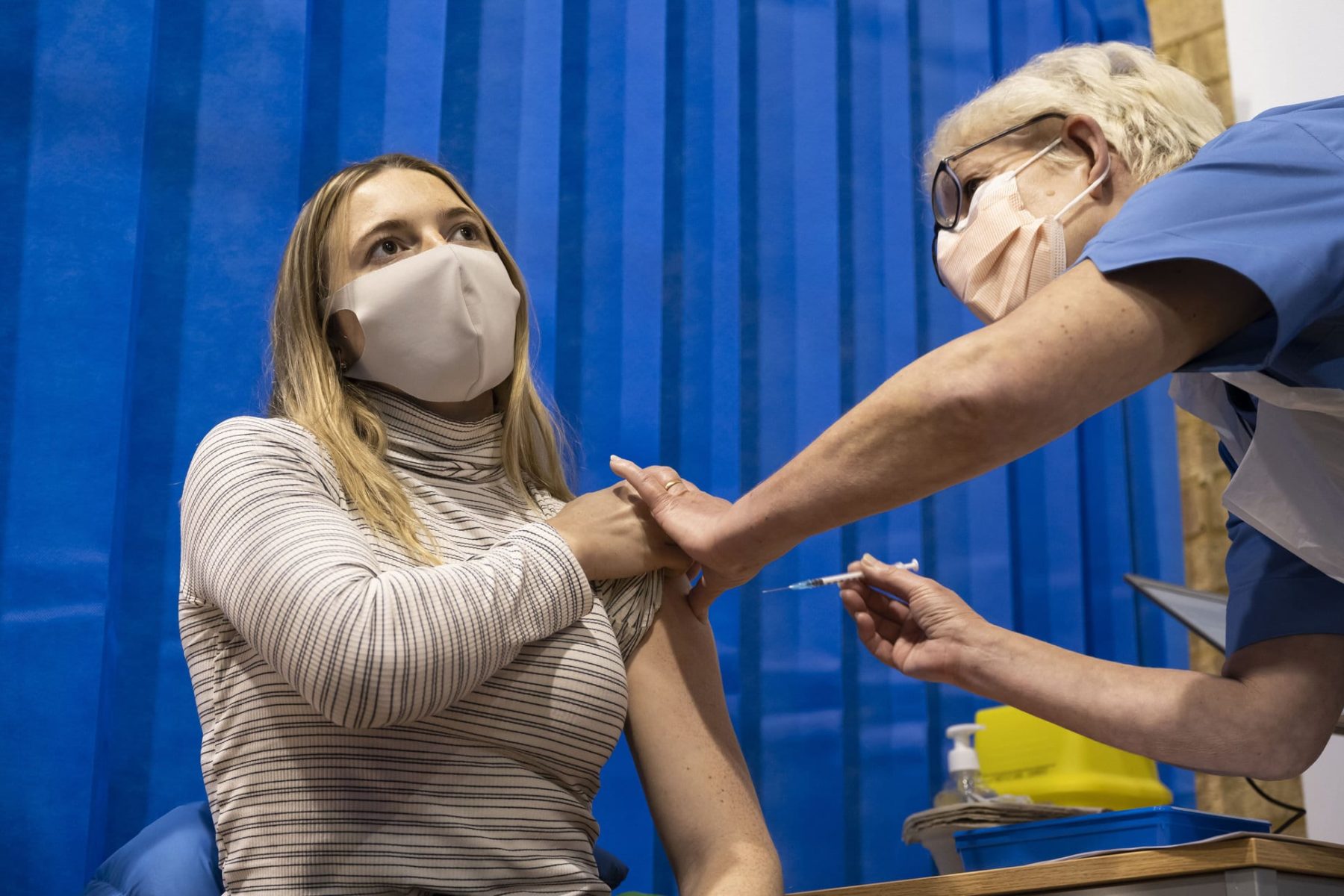 Pregnant Health Care Workers Could Get Pfizer Vaccine After Fda Emergency Authorization
Pregnant Health Care Workers Could Get Pfizer Vaccine After Fda Emergency Authorization
Https Www Fda Gov Media 143352 Download
 Covid 19 Vaccine To Pregnant Women Offered By Chemed In Lakewood Nj
Covid 19 Vaccine To Pregnant Women Offered By Chemed In Lakewood Nj
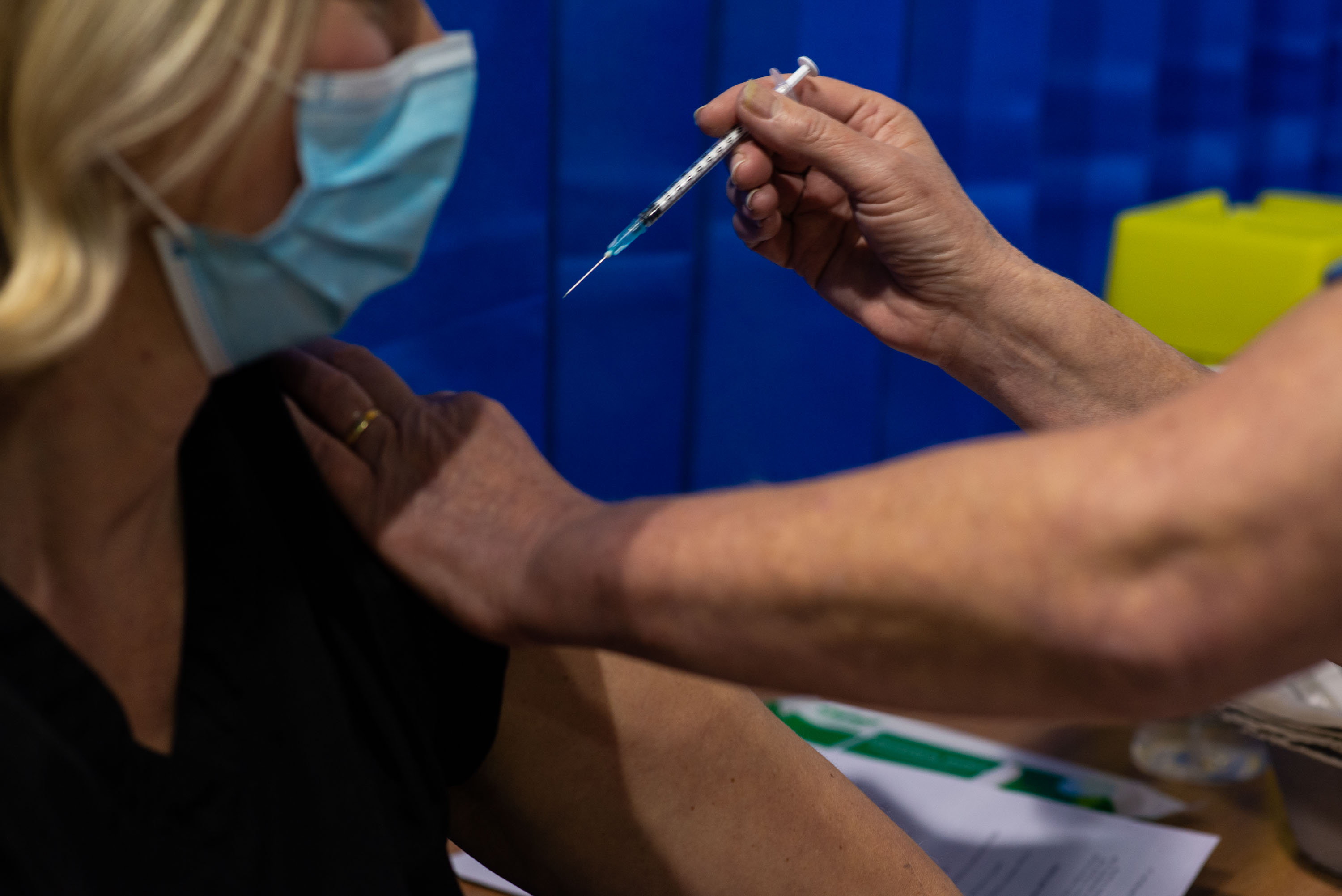 45 Fda Confirms Safety Data And Efficacy Of Pfizer S Covid 19 Vaccine Ahead Of Thursday Meeting
45 Fda Confirms Safety Data And Efficacy Of Pfizer S Covid 19 Vaccine Ahead Of Thursday Meeting
 What Pregnant Women Should Know About The Covid 19 Vaccine Henry Ford Livewell
What Pregnant Women Should Know About The Covid 19 Vaccine Henry Ford Livewell
 Cdc On Twitter Since Covid19 Vaccine Supply Initially Will Be Limited Healthcare Personnel Are Recommended To Be Among The First Vaccinated Healthcare Personnel Can Be At High Risk Of Being Exposed To
Cdc On Twitter Since Covid19 Vaccine Supply Initially Will Be Limited Healthcare Personnel Are Recommended To Be Among The First Vaccinated Healthcare Personnel Can Be At High Risk Of Being Exposed To
Post a Comment for "Covid 19 Vaccine Pregnant Fda"